An exon-skipping therapy used to treat Duchenne.
EXONDYS 51 is used to treat Duchenne in patients who have a confirmed mutation in the dystrophin gene that can be treated by skipping exon 51. EXONDYS 51 was approved under accelerated approval. Accelerated approval allows for drugs to be approved based on a marker that is considered reasonably likely to predict a clinical benefit. EXONDYS 51 treatment increased the marker, dystrophin, in skeletal muscle in some patients. Verification of a clinical benefit may be needed for EXONDYS 51 to continue to be approved.
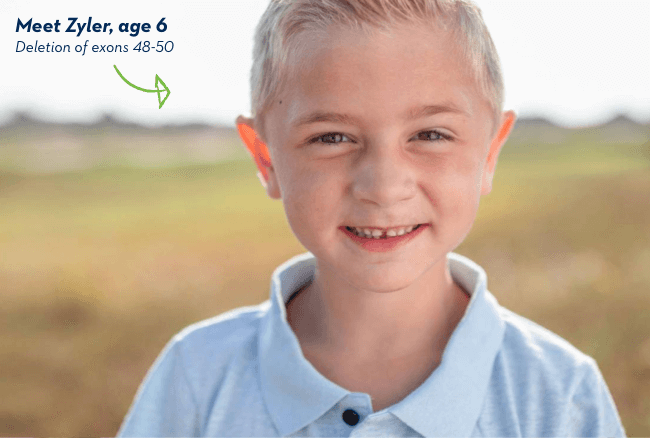
Meet Zyler, age 6
Deletion of exons 48-50
How does it work?
EXONDYS 51 is an exon-skipping therapy that helps the body make a shorter form of the dystrophin protein. Find out more about EXONDYS 51 and the results from its clinical studies.
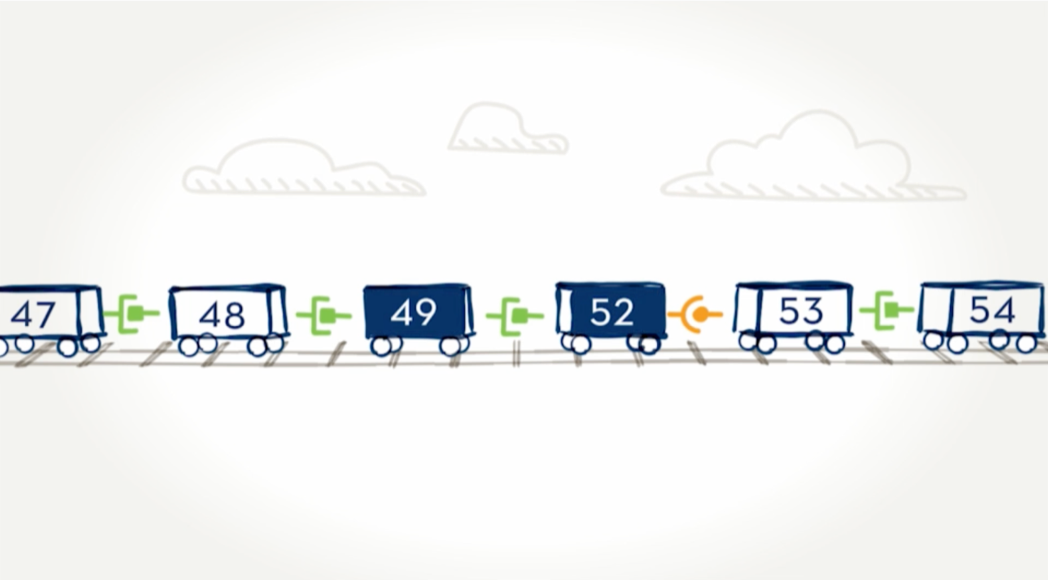
Addressing a lack of dystrophin.
Exon-skipping technologies strive to address the underlying issue with Duchenne—a lack of the protein dystrophin. Many people with Duchenne have a genetic mutation in which one or more exons in the dystrophin gene are missing. This causes errors in the instructions for making dystrophin, leaving the body unable to produce the protein. Exon skipping tells the body to hide an exon next to the missing piece so the whole section can be skipped over and the remaining exons can fit together. EXONDYS 51 helps some boys make a shorter form of dystrophin protein.
Informed decisions.
There are certain risks and side effects associated with EXONDYS 51. As with any medication, you should discuss risks and side effects with your child's doctor.

