
Gaining a deeper understanding of Duchenne muscular dystrophy.
Duchenne muscular dystrophy (DMD) or “Duchenne” is a rare genetic disease that affects mostly boys. Duchenne is caused by a mutation in the gene that’s responsible for the body’s production of dystrophin, a protein that enables muscles to function properly. This mutation causes dystrophin production to be reduced or shut down so muscles become damaged and weaken over time.

The numbers behind Duchenne.

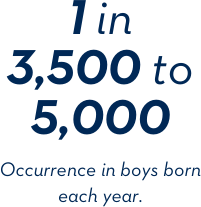

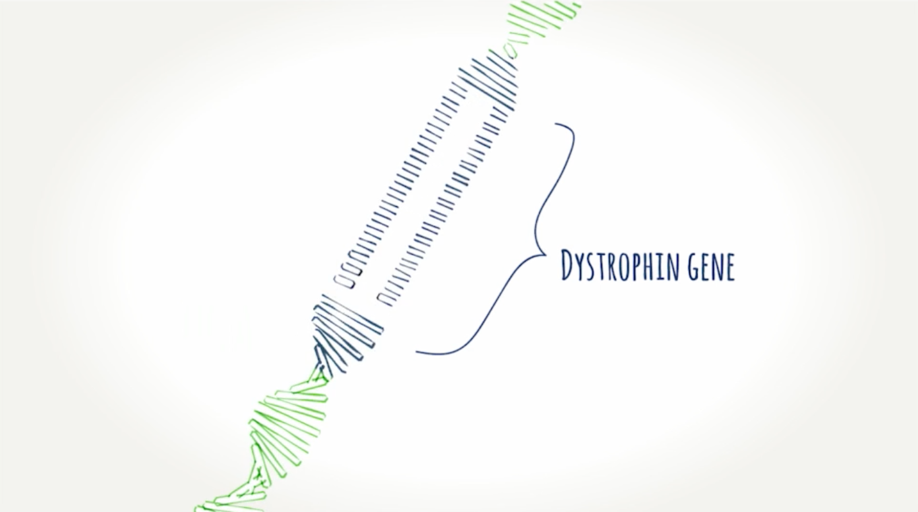
What is dystrophin?
A protein muscles need to work properly. It helps protect the cells in our muscles as we use them. When you don't have enough or any dystrophin, muscles can become damaged and weak. Learn how exon skipping works.
Duchenne muscular dystrophy symptoms.
Children with Duchenne experience developmental delays that may mimic symptoms of other disorders, but the telltale symptoms of Duchenne point to underlying muscle weakness. Parents may first notice that their child isn’t walking by 15 or 16 months of age or is displaying other common symptoms such as:
- An unsteady or waddling gait
- Frequent falls
- Large calves compared to others their age
- Using their arms to walk the torso to a standing position (Gowers' maneuver or Gowers' sign)
- Walking on their toes
- Difficulty running or climbing stairs
- Delays in speech
- Behavior and learning disorders
While these symptoms alone will not confirm a Duchenne diagnosis, they may warrant a visit to your child’s doctor to discuss next steps, including whether a blood test to check their creatine kinase (CK) level is appropriate. To learn more about Duchenne signs and symptoms, visit Parent Project Muscular Dystrophy.
Disease progression.
Duchenne is a progressive disease that weakens muscles. Children with Duchenne go through similar phases, but the rate of progression varies. Because Duchenne affects so many muscles, its signs and symptoms become more widespread throughout the body.
Early phase—In the early phase of Duchenne—through infancy and childhood—a child may have difficulty with typical developmental milestones, such as sitting, walking, or talking.
Transitional phase—As they transition into the later stage of childhood, the child’s hip and leg muscles become weaker and they will have trouble climbing stairs, running, and keeping up with other children. They often walk their hands up their legs to rise from the floor to a standing position—a sign of Duchenne that is commonly known as “Gowers' Maneuver”.
Loss of ambulation—As a child with Duchenne moves into their teen years and muscle strength continues to decline, they tire more easily and may become reliant on a wheelchair. They typically have use of their arms and hands but muscle weakness often leads to difficulties in carrying things like schoolbooks.
Adult phase—Adults with Duchenne experience further decline in muscle function, including the heart and breathing muscles. Breathing becomes increasingly difficult and there may be a greater likelihood of life-threatening heart and lung conditions.

Meet Max, age 13
Deletion of exon 52

Time is of the essence. It's vital that every child gets a genetic test to identify their specific genetic mutation.
Related FAQs
Because Duchenne is a progressive disease, it’s important for your child's doctor to confirm a diagnosis and identify the specific genetic mutation to guide your child's care and treatment. More about the steps to diagnosis.
